Example Report #1
Title
The effects of caffeine on the movement of Physarum polycephalum
Abstract
The effects of caffeine on movement were evaluated in Physarum polycephalum. Physarum uses a signal transduction pathway to control its movement, more specifically a G-protein coupled receptor pathway in which cAMP is the second messenger. The signal pathway is stopped when cAMP is broken down by phosphodiesterase. Caffeine is a known inhibitor of phosphodiesterase, and inhibiting phosphodiesterase activity mimics the stimulation of cAMP production. It was predicted that caffeine treatment would result in increased movement of physarum. The PDA plates were treated with different caffeine concentrations, then physarum was added to the plates and its movement was measured after 24 hours in the light incubator. There were no valid results from this experiment, and this could have been due to the disruption of the resting phase in physarum as the slime mold was moved into and out of the incubator several times.
Introduction
Organisms respond to many different stimuli in the environment around them. Responding to different stimuli allows organisms to gather the resources they need to survive. Physarum is a slime mold that spends most of its life in the plasmodial stage, a yellow flat mass that can grow up to 30 cm in diameter. Physarum lives in damp decomposing leaves and eats ground debris, it engulfs bacteria and other organic debris and then actively transports hydrolytic enzymes into the food vacuoles in order to digest them. Physarum crawls by extending its leading edge using actin microfilaments. It remains a multicellular plasmodium as long as it remains in a dark damp environment, otherwise it moves around looking for food. In the lab physarum is grown on a petri dish using 2% agar and oatmeal flakes, or potato dextrose agar. Physarum displays a complex array of behaviors in response to the stimuli in its environment. These observable behaviors include cytoplasmic streaming, phototaxis movement towards or away from a light source, and chemotaxis movement towards or away from the sensed molecules from the environment [Johnson: 2018].
Physarum uses intracellular signal transduction pathways to respond to its environment. Much of the behavior in physarum is regulated through G-protein coupled receptors and ion-coupled receptors. A specific example of the G-protein coupled receptor pathway in physarum is the cAMP mediated pathway, in which cAMP is used as a second messenger and activates the signalling pathway. During the cAMP mediated signal pathway cAMP diffuses through the cell and binds to several substrates. One enzyme that is commonly activated by cAMP is protein Kinase A (PKA). PKA then phosphorylates many other enzymes which can activate their activity, causing the signal to continue to travel down the pathway. This signal pathway is stopped when cAMP is broken down by phosphodiesterase [Johnson: 2018]. Because phosphodiesterase is responsible for breaking down cAMP, inhibiting phosphodiesterase activity can often produce the same effect as stimulating the production of cAMP. This experiment tested whether caffeine’s inhibition of phosphodiesterase activity in physarum would cause increased movement in response to light. Caffeine was used because it is a known inhibitor of phosphodiesterase activity [Kincaid: 1979]. Caffeine prevents the production of phosphodiesterase, and this mimics the continued production of cAMP. It was therefore predicted that movement in response to the light source would increase due to the continued production of cAMP. To test this, varying concentrations of caffeine were added to PDA plates with physarum, and the effect of caffeine on movement was measured.
Materials and Methods
Preparing Caffeine concentrations:
1ml of 20mM caffeine solution was diluted with 19ml of water to make a 0.05mol solution. And 0.5ml of 20mM caffeine solution was diluted with 19.5ml of water to make a 0.025mol solution.
Preparing the plates:
Three control PDA plates were labeled as control and two squares of 1cmx1cm physarum were added to the center of each plate. The lids were then secured with tape and the plates wrapped in foil, with a 1cmx1cm window cut out at the bottom of each plate. The plates were then labeled again on the outside of the foil.
Three PDA plates were labeled 10mM caffeine, and 5ml of caffeine solution was added and spread around each plate. After 10 mins the excess caffeine solution was blotted off using kimwipes. Two squares of 1cmx1cm physarum were then added to the center of each plate. The lids were then secured with tape and the plates wrapped in foil, with a 1cmx1cm window cut out at the bottom of each plate. The plates were then labeled again on the outside of the foil.
Three PDA plates were labeled 20mM caffeine, and 5ml of caffeine solution was added and spread around each plate. After 10 mins the excess caffeine solution was blotted off using kimwipes. Two squares of 1cmx1cm physarum were then added to the center of each plate. The lids were then secured with tape and the plates wrapped in foil, with a 1cmx1cm window cut out at the bottom of each plate. The plates were then labeled again on the outside of the foil.
All 9 plates were then placed in the light box incubator for 24 hours. After 24 hours the distance of physarum migration was measured in each plate, and the distance as well as the direction of the migration was recorded. A one way ANOVA test was used to compare the means of each group to each other to determine if the concentration of caffeine had a significant effect on the movement in each group.
Results
The results from this experiment were inconclusive as there was no movement in the 10mM Caffeine treatment group, and the 20mM Caffeine treatment group moved the same distance as the control group.
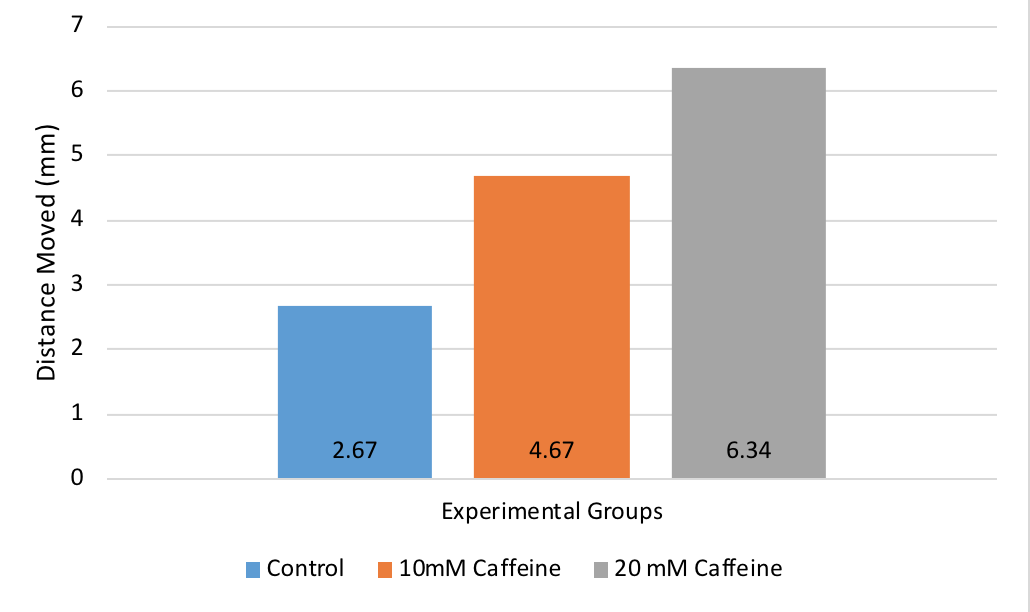
If the experiment had proceeded as expected, then there should have been increased movement in the two caffeine treatment groups, as shown below with the example data of what should have been observed.
The graph shows that the average distance moved was the greatest in the 20mM Caffeine treatment group, an average distance of 6.34mm. The average distance moved was greater in the 10mM Caffeine treatment group than the control group, an average distance of 4.67mm. The treatment group with the highest concentration of caffeine showed the greatest movement, but both caffeine treatment groups had greater movement than the control group.
Discussion
We expected to see greater movement in the two caffeine treatment groups. Caffeine is a phosphodiesterase inhibitor, and this mimics the stimulation of cAMP production [Beavo: 1970]. cAMP activates the signalling pathway and affects the motile behavior of physarum, therefore increased intracellular cAMP concentration should have resulted in increased motility in physarum via enzyme inhibition [Adamatzyky: 2013]. This is shown in the results that show the expected observations for this experiment.
Our results however did not support the hypothesis, as there was no movement in the 10mM Caffeine treatment group, and the 20mM Caffeine treatment group moved the same distance as the control group. A possible explanation as to why the expected results were not obtained could be due to the disruption of the resting phase in physarum. For resting phase to be successfully maintained in physarum, the continued presence of the inducing stimulus is required, and the resting phase is important in the development of physarum [Dove: 1980]. Changing the stimulus that the physarm was exposed to, such as changing the temperature and light exposure, could have disrupted the resting phase.
We moved the physarum into and out of the incubator several times throughout the experiment, and this changed the temperature and also the light intensity that the physarum was exposed to. Exposing the physarum to different temperatures, including cold temperatures in the lab, could have disrupted the normal development of physarum. The temperature in the lab would get colder at nights, and this decrease in temperature could have negatively affected the physarum. A previous study found that microfilament structures in physarum disintegrated in cold temperatures [Furuya: 1986], and this could have inhibited the movement of the physarum in our experiment. Exposing the physarum to different light intensities could have also disrupted the normal development of physarum. The light the physarum was exposed to changed when the plates were wrapped and unwrapped several times throughout the experiment. Physarum undergoes sporulation in response to light [Weaver: 1976], therefore changing the light exposure could have affected the normal development and movement of our physarum.
Literature Cited
- Kincaid, R., Mansour, T. 1979. Cyclic 3’,5’-AMP phosphodiesterase in Physarum polycephalum: II. Kinetic properties. BBA. 588: 342-350.
- Beavo, J., Crofford, J., Hardman, J., Newman E., Rogers, N., Sutherland, E. 1970. Effects of Xanthine Derivatives on Lipolysis and on Adenosine 3’5’ Monophosphate Phosphodiesterase activity. Molecular Pharmacology. 6: 597-603.
- Adamatzky, A., Lacy Costello, B. 2013. Assessing the chemotaxis behavior of Physarum polycephalum to a range of simple volatile organic chemicals. Communicative and Integrative Biology. 6(5).
- Dove, W., Rusch, H. 1980. Growth and differentiation in Physarum polycephalum. New Jersey: Princeton University Press. 157-159.
- Furuya, M., Uyeda, T. 1986. Effects of low temperature and calcium on microfilament structure in flagellates of Physarum polycephalum. Experimental Cell Research. 165: 461-472.
- Weaver, R., Wormington, W. 1976. Photoreceptor pigment that induces differentiation in the slime mold Physarum polycephalum. Biochemistry. 73: 3896-3899.
- Johnson AD. Cell Structure. Biological Principles Laboratory Manual. Dept. Biology, Wake Forest University, Winston-Salem, NC. Vers 16.1 (updated 2018).
Notes For Instructors
Primary Points to Focus On First
- The author gives no clear reason in the Introduction why they think cAMP controls phototactic response.
- The Results section is deeply flawed. They report their observed outcomes, but then do not summarize their data. Instead they show example data for what they had hoped to see.
- The Discussion focuses on what they might have done wrong, not on interpreting what they actually saw.
Other Points of Concern
- The Title could be more informative.
- The Abstract is too detailed.
- The first paragraph of the Introduction states random facts without a clear goal. The second paragraph could be much shorter.
- A lot of the information provided in the first paragraph of the Discussion should have been in the Introduction.
Example Report #2
Title
The Effects of Caffeine on Physarum polycephalum cell signaling in the Cyclic Adenosine 3’,5’-Monophosphate-mediated Pathway
Abstract
All animals use cell signaling to respond to stimuli and react accordingly. Cell signaling is difficult to observe in cells, therefore the protist Physarum polycephalum is used instead. This slime mold spends most of its life in a plasmodial stage in which mass of nuclei within a single plasma membrane will behave as a single organized cell. Physarum uses the cAMP-mediated cell signaling pathway, in which cAMP is the secondary messenger that signals to other molecules, to promote movement and “crawl” along surfaces in search of food. Caffeine has been experimentally determined to inhibit phosphodiesterase which is used in the cAMP pathway to breakdown cAMP. We hypothesized that inhibition of phosphodiesterase would allow cAMP to continue signaling and promote movement of the Physarum. The results of the data supported our hypothesis and, compared to a control sample, caffeine caused a significant change in how much Physarum moved towards food within 24 hours.
Introduction
All animals utilize cell signaling to react to stimuli, coordinate a response via messenger molecules, and make the necessary changes to maintain homeostasis. Due to the difficulty of observing cell signaling within individual animal cells, the protist slime mold Physarum polycephalum is used instead. Physarum spends most of its life cycle in the plasmodial phase. In this stage, the physarum slime mold mass will behave as a single organized cell, or rather, a multinucleated syncytium—a mass of nuclei within a single plasma membrane. During this phase, the mass moves and searches for food by constantly “crawling” with its actin microfilaments. Since the syncytium behaves like a single cell, it is useful to observe how the syncytium reacts to experiments instead of studying individual animal cells. By examining how Physarum reacts to stimuli, it allows for better understanding of how single cells use signal transduction pathways to react external stimuli. One of the major pathways Physarum uses for movement is the Cyclic adenosine monophosphate(cAMP)-mediated pathway. This pathway proceeds by stimuli eventually activating cAMP, the second messenger molecule, which can be promoted or inhibited by different chemical compounds. In the cAMP-mediated pathway, cell signaling stops when phosphodiesterase breaks down cAMP. This lab focuses on how the protist Physarum polycephalum’s cell signaling responds to exposure of caffeine. Caffeine is experimentally known to be an inhibitor of phosphodiesterase (Johnson:2016). Inhibiting phosphodiesterase causes the same response as stimulating production of cAMP since the signaling molecule cannot be broken down(Johnson:2016). Futhermore, Dr. Levin, Dr. Greenberg, and Dr. Wein support this research as they found in their own research that caffeine in high concentrations, significantly increased metabolism and motility of human sperm cells in semen (Levin: 1981). Sperm motility is also attributed to cAMP present in the cells. Their research showed an increase in cellular levels of cAMP after “mixed inhibition” of phosphodiesterase by caffeine (Levin: 1981). We hypothesize that by stimulating Physarum with caffeine, to inhibit breakdown of cAMP and continue signaling, the slime mold will show more growth and migration than normal.
Materials and Methods
First, six water agar plates were obtained and three were labeled “control” and the other three labeled “with caffeine”. Next, 1ml of 20X stock solution caffeine was diluted into 19ml of water to a final concentration of 1X. After mixing the caffeine/water solution, 6ml were added to each plate labeled “with caffeine”. After approximately 20 minutes of waiting for the agar to absorb the solution, excess liquid was gently and carefully dabbed off with Kim lab wipes. Next, using a sterile spatula one approximately 1cm x 1cm square was cut in the middle of each plate. The spatula was sanitized each time by dipping it into an ethanol solution. Being sure to sterilize before each transfer, each square in the experimental and control plates were then filled with a square of the same size from the Physarum stock plate, making sure the Physarum stock square was well colonized by the slime mold and was placed upwards. Finally, about 5-6 oatmeal flakes were scattered at the tops of each of the plates. The plates were the wrapped in aluminum foil and placed in an incubator at 37 C for 24 hours. The following day the growth of the Physarum towards the oatmeal was measured and recorded. A one-tailed T-test will be run to determine the statistical significance of the results.
Results
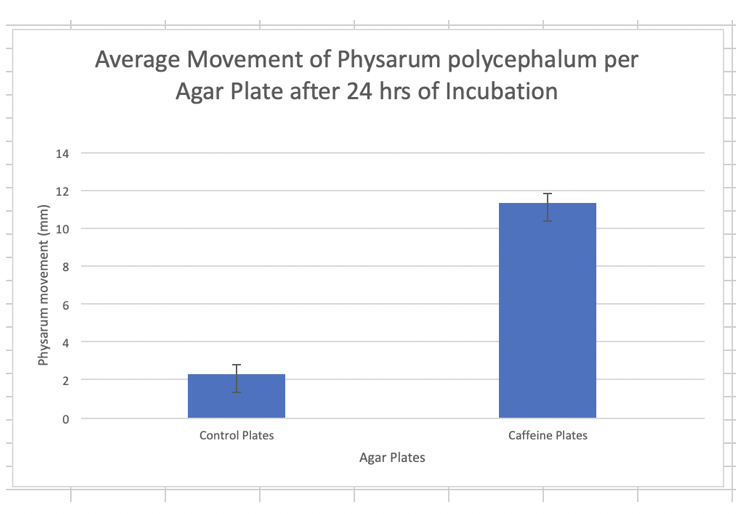
The results show the average growth of Physarum with caffeine (11.33+/- 1.25) was significantly higher than average growth of the control plates (2.33+/-0.47). The one tailed t-test run on the data yielded a P-value of 0.000336297 which showed the results were statistically significant.
Discussion
Cells respond to external stimuli by activating different signal transduction pathways. Since individual cells are difficult to observe, the slime mold Physarum polycephalum can be utilized since its plasmodial life stage is a multinucleated syncytium, which behaves as a single cell would. One of the main signaling pathways that Physarum uses for movement is the cAMP-mediated pathway. This pathway responds to external stimuli and will signal a response by using cAMP as a secondary messenger. This allows for the pathways to be selectively inhibited or promoted. It is experimentally known that caffeine is an inhibitor of phosphodiesterase, the enzyme used in the cAMP-mediated signaling pathway to stop signaling by breaking down cAMP (Johnson:2016). Inhibition of phosphodiesterase thus causes the same response as stimulating cAMP production (Johnson:2016). With this knowledge, we hypothesized that treating Physarum with caffeine would promote more movement since it would block phosphodiesterase and thus prevent the Physarum to stop the signal for movement. The hypothesis was supported by the data of the lab since the Physarum treated with caffeine on average moved more (11.33+/- 1.25mm) than the control Physarum samples (2.33+/- 0.47mm) (Figure 1). Furthermore, the results of the experiment were determined to be statistically significant (p_value=0.000336297) and likely did not occur due to error or chance. The results supported that caffeine was an inhibitor of phosphodiesterase, since the increased movement was a result of intracellular cAMP buildup. The cAMP would have been broken down by phosphodiesterase had it not been inhibited by caffeine, which shares a similar chemical structure to adenosine. This similarity means caffeine likely binds to phosphodiesterase before cAMP can, thus blocking the active site phosphodiesterase uses to act on cAMP. The results of our data were also supported by the research of Drs. Levin, Greenberg, and Wein, which showed an increase in motility of sperm after treatment with caffeine, a “mixed inhibitor” sperm phosphodiesterase (Levin: 1981). It should be noted however, that research by Drs. Brenner and Thoms on the social ameba Dictyostelium discoideum showed that in the cAMP pathways, caffeine did not act on phosphodiesterase, but rather inhibited adenylate cyclase, the enzyme responsible for catalyzing the change of ATP to cAMP (Brenner: 1984). This research offers that caffeine may not be selectively inhibiting phosphodiesterase, and rather may have another mechanism that caused Physarum to have increased movement. For example, caffeine may function by altering intracellular calcium distribution, which, the same mechanism by which the IP3 cell signaling pathway functions (Brenner: 1984). To improve this lab, the Physarum slime mold used for lab should be ensured to be an active strain as to obtain better overall results. Furthermore, intracellular levels of certain molecules could be tracked to better determine by which mechanism caffeine functions on the cAMP pathway. Lastly, more samples could be used to provide more statistically significant data.
Literature Cited
Brenner M, Thoms SD. “Caffeine Blocks Activation of Cyclic AMP Synthesis in Dictyostelium Discoideum.” Developmental Biology, vol. 101, no. 1, Jan. 1984, pp. 136–146.
Johnson AD. Cell Structure. Biological Principles Laboratory Manual. Dept. Biology, Wake Forest University, Winston–Salem, NC. Vers. 16.1 (updated May 1, 2016), pp. 15–16.
Levin RM, Greenberg SH, Wein AJ. “Quantitative Analysis of the Effects of Caffeine on Sperm Motility and Cyclic Adenosine 3’,5’-Monophosphate (AMP) Phosphodiesterase.” Fertility and Sterility, vol. 36, no. 6, 1981, pp. 798–802.
Notes For Instructors
Primary Points to Focus On First
- The results section reports the findings without any reference to the graphed data. Statistics are reported incorrectly.
- Most of the Discussion section simply repeats what was said in the Introduction.
- The author ends the Discussion with a “what we did wrong” section that is not needed. They would do better to describe their next experiment.
Other Points of Concern
- The Title could give some indication of observed outcome.
- The Abstract provides too much background and does not describe their findings.
- The Introduction could be briefer and better focused. The author needs to provide additional cited sources to support their thinking.
- The Methods read like a list of steps from the lab manual; they could be condensed.
- All of the cited sources are quite old. They need to look for more recent information.